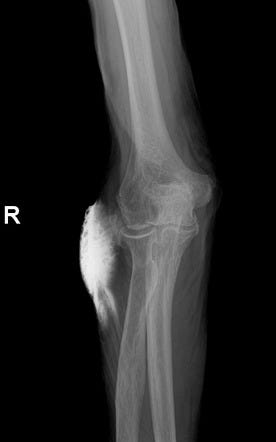
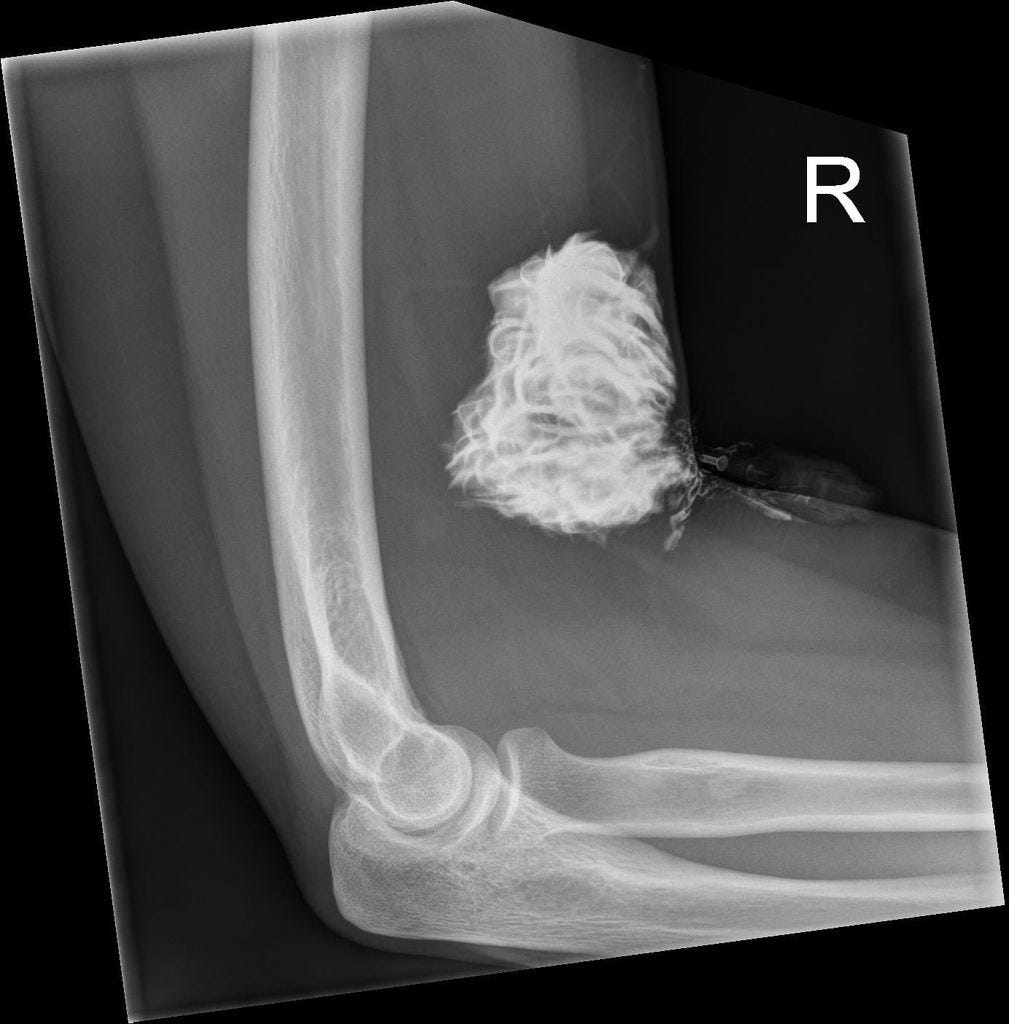
The reported incidence of intravenous (IV) contrast media extravasation in adults and children ranges from 0.1% to 1.2%.
Complains:
Patients complain of initial swelling or tightness, and/or stinging or burning pain at the site of extravasation, some experience little or no discomfort. On physical examination, the extravasation site may be edematous, erythematous, and tender.
In most patients, initial swelling and tenderness resolves within hours to days after the extravasation. The vast majority of patients in whom extravasations occur recover without clinically important sequelae.
However, in some patients, extravasated iodinated contrast media can result in injury to surrounding tissues, particularly the skin, producing an acute local inflammatory response that peaks at 24 to 48 hours. Most of the time there are no lasting complications.
Only rarely will a low-osmolality contrast media (LOCM) extravasation injury proceed to a severe adverse event. Acute tissue injury resulting from extravasation of iodinated contrast media is probably related at least in part to its hyperosmolality.
Severe Complications:
The most commonly reported severe injury after LOCM extravasation is compartment syndrome
. Compartment syndrome results from mechanical compression and is probably more likely to occur after extravasation of larger volumes of contrast media; however, it also has been observed after extravasation of small volumes, especially when these occur in less capacious areas (such as over the ventral or dorsal surfaces of the wrist).
Compartment syndrome may develop soon after an extravasation or result from swelling that sometimes occurs hours after the extravasation.
Less commonly encountered severe injuries include skin ulceration and tissue necrosis. These can occur within hours or days of the extravasation event.
Evaluation:
Ask about symptoms of pain and paresthesias.
A brief examination should be performed and should include assessment of extremity tenderness, swelling, erythema, paresthesia, active and passive range of motion of the fingers, and perfusion.
Management:
Extravasations that resolve rapidly probably need no treatment other than brief observation and discharge instructions.
More symptomatic extravasations may be treated with extremity elevation above the level of the heart (to decrease capillary hydrostatic pressure and thereby promote resorption of extravasated fluid and either warm or cold compresses that an outpatient may continue intermittently at home until symptoms resolve, along with discharge instructions, or may be continued on the wards for inpatients.
Aspiration is not recommended.
Topical application of silver sulfadiazine ointment and steroid cream three to four times daily has been recommended by some as an approach to soothe irritated skin, reduce inflammation, and to prevent infection should any blistering occur, although the efficacy of this treatment is unknown.
There is no consistent evidence that local injection of potentially therapeutic agents, such as corticosteroids or hyalurinidase is beneficial.
Surgical Consultation:
Urgent surgical consultation should be obtained whenever there is concern for a severe extravasation injury.
Although consultation can prolong length of stay, it should be obtained for any patient in whom one or more of the following extravasation-related signs or symptoms develops: severe pain; progressive swelling or pain; altered tissue perfusion as evidenced by decreased capillary refill; change in sensation in the affected limb; worsening passive or active range of motion; and skin ulceration or blistering.
Because of this, surgical consultation should be based on signs and symptoms rather than an absolute volume threshold. If the patient is asymptomatic or has only mild symptoms, appropriate evaluation and clinical follow-up are usually sufficient.
Discharge the patients:
Outpatients who have suffered contrast media extravasation should be released from the radiology department only after an initial period of observation, provided the radiologist is satisfied that any signs and symptoms that were present initially have improved or that new symptoms have not developed during the observation period.
Clear instructions should be given to the patient to seek additional medical care for severe pain, progressive pain, numbness or tingling, diminished range of motion (active or passive), skin ulceration, or other neurologic or circulatory symptoms.
This is because initial symptoms of a serious compartment syndrome may be absent or relatively mild (such as limited to the development of focal paresthesia).
Patients who are at increased risk of extravasation:
Extravasations are more common in patients who cannot communicate effectively (e.g., the elderly, infants and children, and patients with altered consciousness), severely ill or debilitated patients, and patients with abnormal circulation in the limb to be injected.
Patients with altered circulation include those with atherosclerotic peripheral vascular disease, diabetic vascular disease, Raynaud’s disease, venous thrombosis or insufficiency, or prior radiation therapy or extensive surgery (e.g., axillary lymph node dissection or saphenous vein graft harvesting) in the limb to be injected.
Women may have a mild increased risk of extravasation.
Some of these conditions are systemic and cannot be avoided by choosing a different injection site.
Certain intravenous access sites (e.g., hand, wrist, foot, and ankle) are more likely to result in extravasation and should be avoided, when possible.
However, use of these alternate injection sites may be necessary due to lack of availability of the more traditional locations.
Flow rates of up to 3 and 5 mL/sec can be safely achieved through 22 gauge and 20 gauge intravenous catheters, respectively, in the vast majority of patients, provided that there is no increased resistance or pain during a rapid test injection.
Patients injected with more viscous contrast material may be more likely to have extravasations than are patients injected with less viscous contrast material. This effect may be mitigated for viscous media by extrinsic warming to human body temperature prior to injection.
Prevention:
Methods for reducing the risk of extravasation include: meticulous intravenous line insertion technique, using angiocatheters instead of butterfly catheters, confirming position by aspirating blood (although failure to aspirate blood does not exclude the possibility of proper catheter location), flushing an inserted catheter with a test injection of saline to ensure proper flow into the accessed vein, and carefully securing the inserted catheter.
Low-risk intravenous line insertion sites are preferred when feasible. If not feasible, higher risk sites may be considered depending on the risks and benefits of administrating contrast media for the examination indication.
Use of a preliminary saline flush to assess injection pressure prior to contrast media administration has been advocated by a few investigators; however, this has not been adopted by most institutions.
Some have recommended use of a hand-held alarm, which the patient can press should any new symptoms develop.
Interestingly, the practice quality improvement project created by the ACR to assist practices in identifying improvements that could be made to reduce the frequency of extravasations did not find that any significant improvement could be achieved, even when risk factors for extravasations were identified and attempts were made to reduce the extravasation risk in advance.